RefluxStop Surgery for Acid Reflux (GERD) in Europe
Acid reflux (GERD) isn’t just an occasional annoyance anymore. It’s a high-burden condition that is on the rise worldwide. Estimates indicate that the global prevalence of GERD was 825.6 million in 2021.[1]

For many people, treatment starts with lifestyle changes and medicines, especially proton pump inhibitors (PPIs). But meds don’t work well for everyone.[2] Some patients also want an option that reduces long-term dependence on daily tablets. That’s where novel approaches, such as RefluxStop surgery, come into play.[3]
What Is The RefluxStop Procedure?
RefluxStop is a minimally invasive (keyhole) anti-reflux operation in which a surgeon implants a small, passive device in the upper part of the stomach.[4][5] The place is close to where the esophagus meets the stomach (the gastroesophageal junction).[6]
 The goal is to treat gastroesophageal reflux disease (GERD) while maintaining a swallowing mechanism as natural as possible.[5][6] This is possible because the procedure does not “wrap” the stomach around the esophagus, as in a classic Nissen fundoplication.[6]
The goal is to treat gastroesophageal reflux disease (GERD) while maintaining a swallowing mechanism as natural as possible.[5][6] This is possible because the procedure does not “wrap” the stomach around the esophagus, as in a classic Nissen fundoplication.[6]
The implant used by surgeons is produced by Implantica AG, a medtech company behind RefluxStop (a CE-marked device in Europe).[5]
How Does It Work?
Think of reflux as a problem of position and stability at the “valve area” between the esophagus and stomach.[7] In many patients with GERD, the gastroesophageal junction can slide upward toward the chest. That makes reflux easier.[8][9]
RefluxStop works as a mechanical support or anchor.[8] It helps keep the lower esophageal sphincter (LES) (improving the function) and junction in a more natural position below the diaphragm. This supports the body’s own anti-reflux barrier.[6][8]
Importantly, it aims to do this without encircling or squeezing the food passageway, which can contribute to gas-bloat or swallowing issues in some traditional anti-reflux surgeries.[5][6]
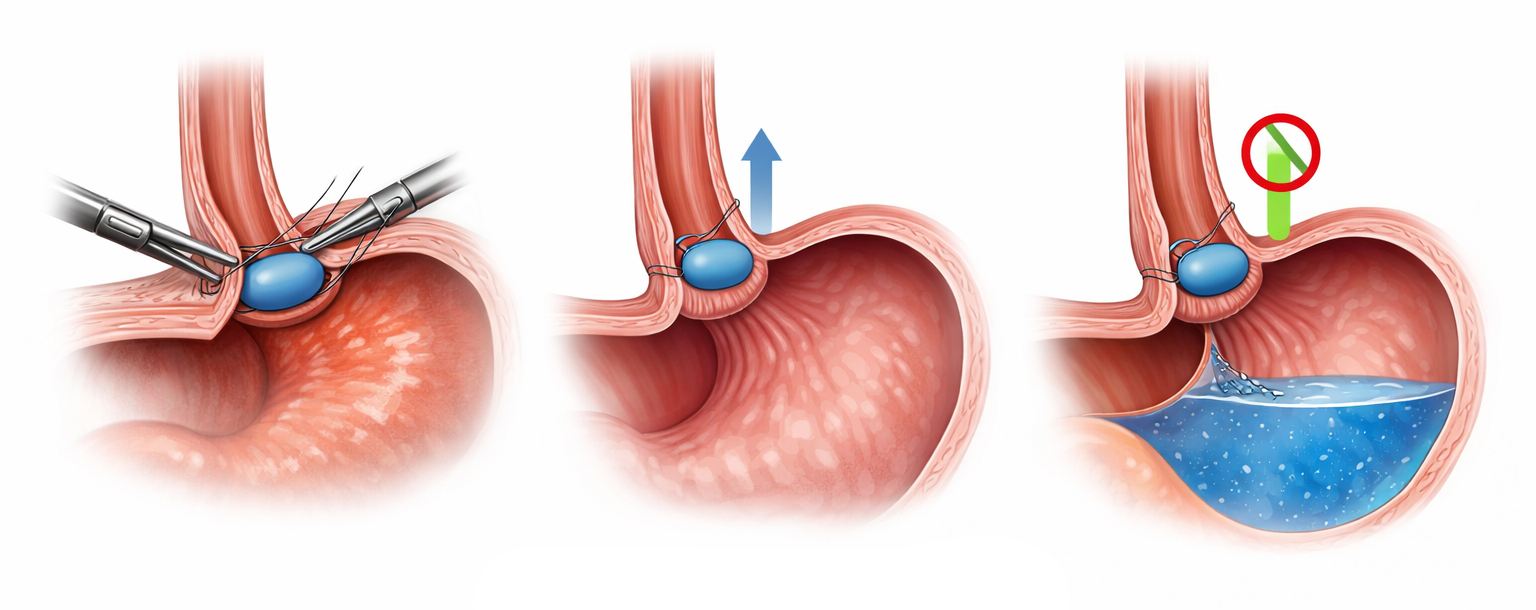
What The Surgery Is Like
RefluxStop surgery is usually done under general anesthesia with several small abdominal incisions (laparoscopy), sometimes using a robotic-assisted technique.[9][10] The surgeon typically frees and repositions the gastroesophageal junction as needed, and often repairs a small hiatal hernia at the same time if present.[10][11] The RefluxStop implant is then fixed to the upper stomach wall to prevent the junction from moving upward.
The laparoscopic GERD surgery with RefluxStop typically takes 1-1.5 hours.[12] Some cases might require a longer duration, depending on the anatomy and whether extra repair is needed.[13]
In some European centers, it’s treated as a day case with a short stay in the clinic, whereas more complex cases (e.g., large hernia repair) may involve several days in the hospital.[9][13]
Who Is a Good Candidate?
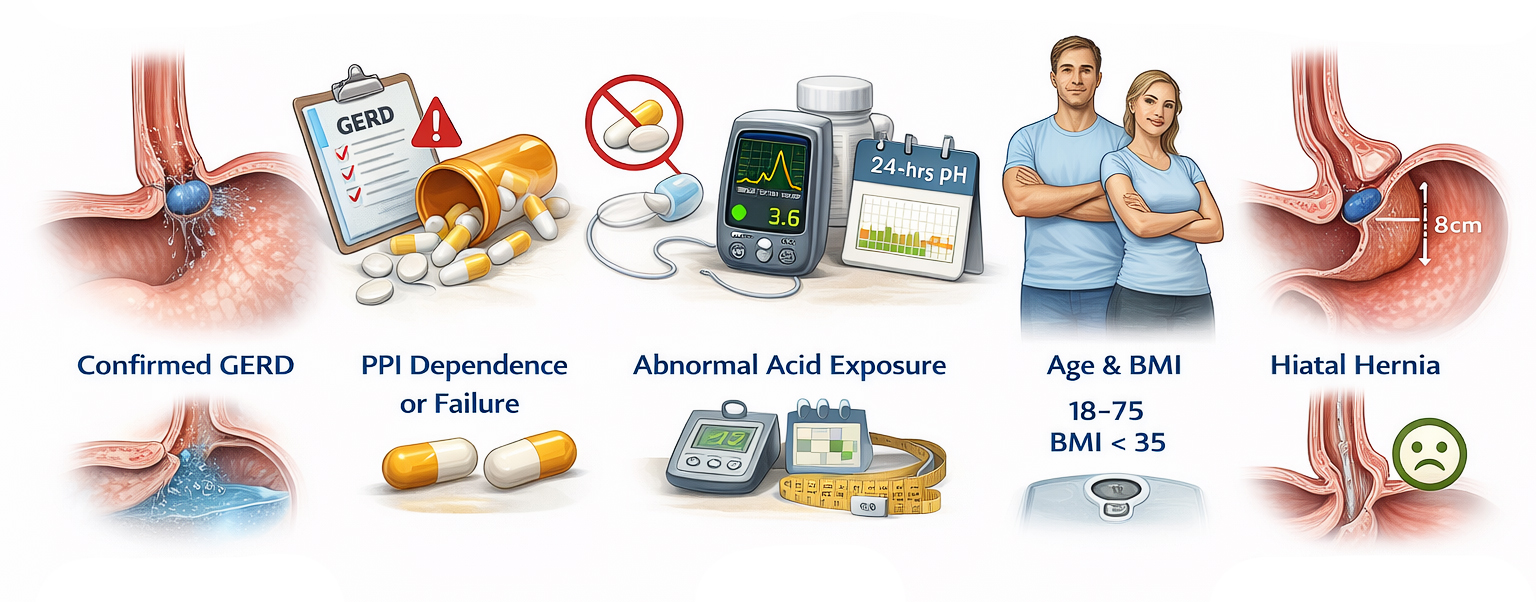
Because this is a relatively new technology, candidates must meet specific clinical criteria established through rigorous preoperative testing. General candidates for a minimally invasive anti-reflux surgery with RefluxStop in Europe typically meet the following profile:
- Confirmed GERD. Documentation of chronic symptoms (heartburn, regurgitation) for at least 6 months.[5]
- PPI Dependence or Failure. Patients who either have "GERD refractory to medication" (symptoms persist despite Proton Pump Inhibitors) or who wish to stop long-term medication due to side effects.[12]
- Abnormal Acid Exposure. A 24-hour pH monitoring test must prove excessive acid exposure (typically pH < 4 for ≥ 4.5% of the time) while off medication.[5]
- Age & BMI. Generally aged 18 to 75 years with a Body Mass Index typically under 35.[5]
- General Health. Are medically fit for laparoscopic surgery and have anatomy that matches the technique.[14]
- Hiatal Hernia. Many candidates have a small hiatal hernia. Newer 2024–2025 clinical data has expanded use to larger hernias (up to 8–10 cm), provided they are reducible during surgery.[5][8]
- Esophageal Motility. It is often a preferred choice for patients with ineffective esophageal motility. The device is safer for patients with "weak" swallowing muscles who might otherwise experience severe blockage with a traditional wrap.[11][13]
In clinical practice, candidacy is determined after a specialist workup. That commonly includes endoscopy, reflux testing (such as pH monitoring), and, often, esophageal motility testing.[14] Choosing the right procedure depends on what’s truly driving your symptoms.
Best Hospitals & Surgeons in Europe for RefluxStop
Europe has numerous clinics offering surgery to treat GERD, but not all hospitals have the same level of experience with the RefluxStop operation. The safest approach is to look for a team of high-volume foregut surgeons that treats GERD every day, performs a full diagnostic work-up, and can clearly explain why RefluxStop fits your case better than alternatives.
European Reflux Centers
Finding the right centre definitely matters. Patients typically prefer a specialized surgical unit or a large-volume general surgery department with a strong section in GERD surgery. Find out the leading European reflux centers at a glance.
Surgeons Offering RefluxStop
For patients whose main priority is an experienced team, check our vetted RefluStop surgeons across Europe. Explore doctors’ profiles to see more details, reviews, and credentials.
RefluxStop Surgery Cost in Europe
When people search for refluxstop cost, they’re usually trying to answer two questions fast: How much will it actually cost me? and Why does the price change so much from one clinic to another? Both are fair questions because RefluxStop surgery isn’t a single fixed-price service across Europe.
The total refluxstop cost can include more than the operation itself: diagnostic tests, surgeon and anesthesia fees, hospital stay, possible hiatal hernia repair, and the implant. In Europe, pricing varies substantially across countries due to different healthcare systems, clinic pricing models, and local costs.
Comparative Cost Table by Countries
| Country | Price range |
|---|---|
| Germany | From €24,800 |
| Italy | €18,900 - €27,300 |
| Spain | From €25,000 |
| Switzerland | €26,700 - €48,000 |
| Austria | From €21,850 |
| United Kingdom | From €15,100 |
| Sweden | From €27,000 |
| Norway | From €19,500 |
Even within the same country, the final refluxstop operation cost can vary depending on the surgeon’s experience, the hospital level (private vs. university), and the complexity of your case. That’s why comparing offers only makes sense when you know exactly what’s included in the quote.
RefluxStop vs Nissen Fundoplication vs LINX
Patients who explore a surgery for GERD acid reflux rarely start with only one option in mind. They usually compare GERD surgery options because the disease is not a “one-size-fits-all”. Choosing between reconstruction and device-based anti-reflux surgery is not only about stopping acid exposure; it is also about long-term function, comfort while eating, and what daily life looks like after treatment.
This comparison is important because the trade-offs patients worry about are practical and immediate. Below, we have provided a side-by-side comparison of typical operations for acid reflux, so patients can find the "best surgery for GERD" in specific scenarios.
RefluxStop vs LINX
 It is a comparison within device-based anti-reflux surgery, but the mechanics differ: the LINX Reflux Management System augments the lower oesophageal sphincter with a magnetic ring around the oesophagus, while the RefluxStop implant is positioned externally to stabilise the reconstructed gastro-oesophageal junction.[15]
It is a comparison within device-based anti-reflux surgery, but the mechanics differ: the LINX Reflux Management System augments the lower oesophageal sphincter with a magnetic ring around the oesophagus, while the RefluxStop implant is positioned externally to stabilise the reconstructed gastro-oesophageal junction.[15]
Examining 158 patients after the RefluxStop procedure, PPI meds use decreased by 96.3%, and new-onset dysphagia was 3.2% without the need for dilation.[16]
At the same time, for LINX, a 5-year pilot study reported GERD improving from 25.7 to 2.9, with 87.8% discontinuing PPIs and 70% achieving pH normalisation among those tested.[15] Longer follow-up data reported PPI discontinuation in 79% and pH normalisation in 89% of those tested.[17]
When patients focus on side effects in RefluxStop vs LINX, dysphagia is often central. In a 380-patient group after LINX, 15.5% had persistent dysphagia, 31% required at least one dilation, and 1.8% required device removal specifically for dysphagia.[18]
RefluxStop vs Nissen Fundoplication
In this scenario, patients are weighing an implant-supported anatomic repair against a full 360° wrap.
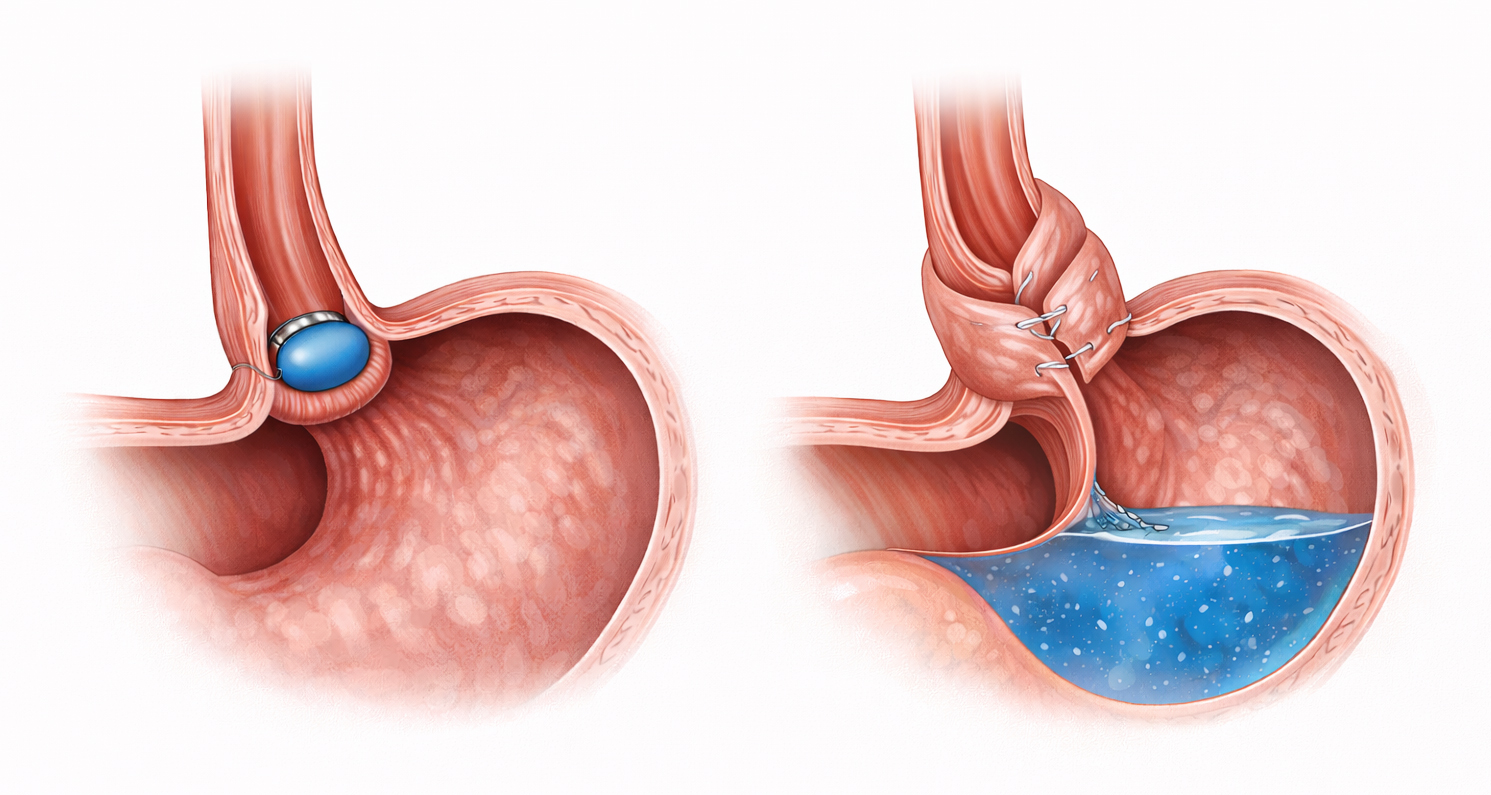 In a German group of 158 RefluxStop cases with follow-up, median GERD-HRQL improved by 90.9% (to 2 from 22), PPI use decreased by 96.3%, and new-onset dysphagia occurred in 3.2% with no postoperative dilations; hiatal hernia recurrence was 1.3%, and device migration was 1.3%.[16]
In a German group of 158 RefluxStop cases with follow-up, median GERD-HRQL improved by 90.9% (to 2 from 22), PPI use decreased by 96.3%, and new-onset dysphagia occurred in 3.2% with no postoperative dilations; hiatal hernia recurrence was 1.3%, and device migration was 1.3%.[16]
By contrast, long-term data for laparoscopic Nissen fundoplication for over 10 years report postoperative PPI use in 24%, dysphagia in 26%, gas-bloating in 53%, symptom recurrence in 17%, and reoperation in 6%, despite high satisfaction (87%).[19]
Patient Results & Long-Term Outcomes
Long-term reflux control is the key question for anyone considering RefluxStop surgery, because early symptom relief matters far less than whether results remain stable over the years.
Patients also ask, can surgery fix GERD in a lasting way, and the only credible answer comes from follow-up data that tracks symptom scores, medication use, objective reflux testing, and durability events over time.
Below, we summarize published study results to show the exact success rates and overall effectiveness reported for RefluxStop.
| Category | Clinical metric | Outcome |
|---|---|---|
| Symptom control | GERD-HRQL Score | 90% reduction in symptoms |
| Daily PPI usage | 97.9% of patients PPI-free | |
| Daily regurgitation | 97.6% resolution rate | |
| Objective data | Acid exposure (pH < 4) | 90.4% improvement in pH |
| Hiatal hernia recurrence | No re-herniation at 5 years | |
| Mechanical safety | Device migration | Zero migrations in pivotal study |
| Device explantation | No devices removed for failure | |
| Side effects | Bothersome dysphagia | Significant reduction in swallowing issues up to 2% |
| Gas-bloat syndrome | Rare or resolved | |
| Patient experience | Patient satisfaction | 95.5% satisfied |
Data used for the result table above.[3][20][13][21]
Regulatory Details
When you’re dealing with an implant, certification isn’t a “nice-to-have.” It’s proof that a device has gone through a formal regulatory process for safety, performance, and manufacturing quality, and that there’s a system for reporting issues and monitoring real-world outcomes.
EU Approval
In Europe, RefluxStop is marketed as a CE-marked medical device. Implantica (the manufacturer) states that RefluxStop has been CE marked in Europe since 2018.[22] Find the list of countries where it is currently approved (including Germany, the UK, Switzerland, Spain, Italy, France, Austria, Sweden, and Norway).
RefluxStop FDA Approval
In the United States, the situation is different. RefluxStop is not available for sale in the USA, and the device is pending U.S. FDA approval.[23] Don’t assume availability in the U.S. until FDA approval is formally granted and the device is marketed there.
FAQ
Is RefluxStop available in the US?
No. RefluxStop is not currently available in the U.S and is not FDA-approved.
Is RefluxStop available in every EU country?
No. “CE-marked” does not automatically mean it’s offered everywhere. Availability depends on local rollout, the availability of trained surgeons, and hospital procurement.
Can I travel to Europe for RefluxStop surgery if it’s not offered in my country?
Often, yes. Many patients travel within Europe for reflux surgery. The key is to confirm you’re a candidate after proper diagnostics, and the clinic can support follow-up once you return home.
Do I need specific tests before I can book RefluxStop in Europe?
Usually yes. Most serious reflux centers require objective evidence of GERD (typically endoscopy and reflux testing, such as pH monitoring, sometimes motility testing). This avoids “wrong procedure, wrong patient.”
Can RefluxStop be done if I have a hiatal hernia?
It depends on size and anatomy, but nowadays hernias up to 8-10cm can be considered. Some patients undergo hernia repair during the same operation.
Why can the cost vary so much across Europe?
Pricing varies by country, hospital type (private vs. public), surgeon fees, anesthesia, hospital stay, diagnostics, and whether additional repair is required. Always ask what the quote includes.
References
- Xie, F., Yang, B., Yan, Z., Shen, Y., Qin, H., Chen, L., et al. (2025, November 5). Global temporal trends and projections of gastroesophageal reflux disease prevalence: Age-period-cohort analysis 2021. PLOS ONE, 20(11), e0334396. doi:10.1371/journal.pone.0334396. Retrieved February 2026.
- Katz, P. O., Dunbar, K. B., Schnoll-Sussman, F. H., Greer, K. B., Yadlapati, R., & Spechler, S. J. (2022, January 1). ACG Clinical Guideline for the Diagnosis and Management of Gastroesophageal Reflux Disease. The American Journal of Gastroenterology, 117(1), 27–56. doi:10.14309/ajg.0000000000001538. Retrieved February 2026.
- Harsányi, L., Kincses, Z., Veselinović, M., Zehetner, J., & Altorjay, Á. (2025, July 22). Five-year clinical outcomes of RefluxStop surgery in the treatment of acid reflux: a prospective multicenter trial of safety and effectiveness. Surgical Endoscopy, 39, 6163–6179. doi:10.1007/s00464-025-11979-9. Retrieved February 2026.
- National Institute for Health and Care Excellence (NICE). (2025, June 3). Laparoscopic insertion of an inactive implant for gastro-oesophageal reflux disease. HealthTech guidance, HTG749. Retrieved February 2026.
- Bjelović, M., Harsányi, L., Altorjay, Á., Kincses, Z., & Forsell, P. (2020, July 20). Non-active implantable device treating acid reflux with a new dynamic treatment approach: 1-year results. BMC Surgery, 20, 159. doi:10.1186/s12893-020-00794-9. Retrieved February 2026.
- Harsányi, L., Kincses, Z., & Altorjay, Á. (2024, December 19). Acid Reflux Management with the RefluxStop Implant: A Prospective Multicenter Trial with 3-Year Outcomes. Digestive Diseases and Sciences, 70, 665–674. doi:10.1007/s10620-024-08788-w. Retrieved February 2026.
- Kahrilas, P. J. (1997, September 1). Anatomy and physiology of the gastroesophageal junction. Gastroenterology Clinics of North America, 26(3), 467–486. doi:10.1016/S0889-8553(05)70307-1. Retrieved February 2026.
- Borbély, Y., Kroell, D., Gerber, S., et al. (2025, May 3). A safety and effectiveness evaluation of RefluxStop in the treatment of acid reflux comparing large and small hiatal hernia groups: results from 99 patients in Switzerland with up to 4-years follow-up. Hernia, 29, 156. doi:10.1007/s10029-025-03339-2. Retrieved February 2026.
- University Hospital Southampton (UHS). (2024, June 18). Hospital Trust first in UK to implant novel device to treat acid reflux using robotic surgery. Retrieved February 2026.
- National Institute for Health and Care Excellence (NICE). (2025, June 3). 2 The condition, current treatments and procedure. (HealthTech guidance HTG749). Retrieved February 2026.
- Fringeli, Y., Linas, I., Kessler, U., et al. (2024, February 29). Short-term results of laparoscopic anti-reflux surgery with the RefluxStop device in patients with gastro-esophageal reflux disease and ineffective esophageal motility. Langenbeck’s Archives of Surgery, 409, 78. doi:10.1007/s00423-024-03264-5. Retrieved February 2026.
- Fringeli, Y., Linas, I., Kessler, U., & Zehetner, J. (2024, July 15). Exploring the feasibility and safety of laparoscopic anti-reflux surgery with the new RefluxStop™ device: a retrospective cohort study of 40 patients. Swiss Medical Weekly, 154, 3365. doi:10.57187/s.3365. Retrieved February 2026.
- Feka, J., Saad, M., Boyle, N., et al. (2024, July 4). Multicentric short term and safety study of ineffective esophageal motility patients treated with RefluxStop device. Scientific Reports, 14, 15425. doi:10.1038/s41598-024-65751-5. Retrieved February 2026.
- Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). Multi-Society Consensus Conference and Guideline on the Treatment of Gastroesophageal Reflux Disease (GERD). Retrieved February 2026.
- Saino, G., Bonavina, L., Lipham, J. C., Dunn, D., & Ganz, R. A. (2015, October). Magnetic Sphincter Augmentation for Gastroesophageal Reflux at 5 Years: Final Results of a Pilot Study Show Long-Term Acid Reduction and Symptom Improvement. Journal of Laparoendoscopic & Advanced Surgical Techniques A, 25(10), 787–792. doi:10.1089/lap.2015.0394. Retrieved February 2026.
- Elshafei, M., & Lehmann, T. (2024, November 13). BN O03 - Pooled two-year results of the novel RefluxStop implantable device in management of gastroesophageal reflux disease in Germany: Retrospective analysis. BJS, 111(Supplement_9), znae271.022. doi:10.1093/bjs/znae271.022. Retrieved February 2026.
- Ferrari, D., Asti, E., Lazzari, V., Siboni, S., Bernardi, D., & Bonavina, L. (2020, August 13). Six to 12-year outcomes of magnetic sphincter augmentation for gastroesophageal reflux disease. Scientific Reports, 10, 13753. doi:10.1038/s41598-020-70742-3. Retrieved February 2026.
- Ayazi, S., Zheng, P., Zaidi, A. H., Chovanec, K., Chowdhury, N., Salvitti, M., et al. (2020, January). Magnetic Sphincter Augmentation and Postoperative Dysphagia: Characterization, Clinical Risk Factors, and Management. Journal of Gastrointestinal Surgery, 24(1), 39–49. doi:10.1007/s11605-019-04331-9. Retrieved February 2026.
- Principe, J., Angeramo, C. A., Barros Sosa, J., Baz Gallego, J. J., Herbella, F. A. M., Patti, M. G., et al. (2025, December 18). Long-Term (>10 Years) Outcomes of Laparoscopic Nissen Fundoplication: A Systematic Review and Meta-Analysis. Annals of Surgery. doi:10.1097/SLA.0000000000007000. Retrieved February 2026.
- Harsányi, L., Kincses, Z., Veselinović, M., Zehetner, J., et al. (2025, June 20). Food passageway-related sequelae in the RefluxStop prospective multicenter trial: patient-centric outcomes of dysphagia, odynophagia, gas-bloating, and inability to belch and/or vomit at 5 years. Surgical Endoscopy, 39, 4615–4627. doi:10.1007/s00464-025-11818-x. Retrieved February 2026.
- Zehetner, J., Niebuhr, N., Linas, I., Kessler, U., & Fringeli, Y. (2026, February). Laparoscopic antireflux surgery with the RefluxStop implant for severe sufferers with complex disease: a retrospective study of the first 100 patients with 12-month follow-up at an early adopter institution. Journal of Gastrointestinal Surgery, 30(2), 102293. doi:10.1016/j.gassur.2025.102293. Retrieved February 2026.
- Implantica. (2018, August 8). Implantica receives CE Mark approval for RefluxStop, a potential paradigm shift in the treatment for acid reflux. Retrieved February 2026.
- Implantica. (2025, September 17). Implantica’s RefluxStop sparks strong excitement at the American Foregut Society meeting ahead of pending US FDA approval. Retrieved February 2026.


















